|
home | what's new | other sites | contact | about |
|||||
|
Word Gems exploring self-realization, sacred personhood, and full humanity
Dr. Stephen C. Meyer's
An Investigation of the 'Cambrian Explosion'
return to "Evolution" main-page
from https://stephencmeyer.org/2013/10/24/does-lightning-fast-evolution-solve-the-cambrian-enigma/
Last month, as ENV noted earlier, the science news media were abuzz about a new paper in Current Biology, “Rates of Phenotypic and Genomic Evolution During the Cambrian Explosion,” by Michael Lee and his colleagues. The paper purports to show that rates of evolutionary change during the Cambrian period were elevated — however not to such an extent as to upset the neo-Darwinian understanding of evolution via natural selection and random mutation. The insistence that these findings pose no problem for Darwin’s theory has been a major theme in media commentary about the paper. Reporting on its conclusions, Live Science explains that “scientists have figured out just how quickly evolution was occurring during evolution’s ‘big bang.’ And it was fast by most measures, five times quicker than occurs today.” Dr. Lee is quoted as saying that he finds this “perfectly consistent with Darwin’s theory of evolution.” A piece at Science Now, the journal Science‘s news desk, goes further, assuring readers that these results not only are reconcilable with but positively vindicate evolution by natural selection: “Their finding — that the rate of change was high, but still plausible — may put Darwin’s fears to rest.” This curious reference to the “fears” of the long deceased Darwin may reflect an implicit acknowledgment of the challenge posed by Darwin’s Doubt. If so, it would not be the first time that the science media or a science journal has responded to the arguments in the book without referring to it by name. As Casey Luskin and David Klinghoffer have pointed out, this has become somewhat of a pattern of late. As Luskin notes, the paper by Lee et al. itself “makes reference to ‘opponents of evolution,’ and critiques a very Meyer-esque argument.” So then, let’s take a closer look.Does this paper in Current Biology explain the explosive origin of animal life in the Cambrian period? In other words, does it identify a causal mechanism capable of producing the novel animal forms and biological information that arose during Cambrian? Does it, thus, provide a refutation of the main arguments of Darwin’s Doubt? It does not. Instead, by using the term evolution in equivocal ways, the authors end up presenting the problem of the Cambrian explosion (the rapid emergence of new forms of animal life) as it own solution (which they simply re-describe as the rapid “evolution” of new forms of animal life). To understand this exercise in rhetorical legerdemain, we need to recall that “evolution” can be defined in several different ways. The term may refer to: (1) the fact of biological change over time, (2) the theory of universal common descent (which implies continuous biological change over time), or (3) the claim that natural selection acting on random variations and mutations is sufficient to cause the change that has occurred in the history of life, including major morphological innovation such as occurred during the Cambrian explosion. As I observe in Darwin’s Doubt, given a Darwinian commitment to universal common descent — “evolution” in the second sense — the absence of discernable ancestors in the Precambrian fossil record is mysterious. However my main argument in the book concerns the inadequacy of “evolution” in the third sense. In particular, I argue (for five separate reasons) that the mutation/natural selection mechanism lacks the creative power to produce the origin of the new forms of animal life in the Cambrian period. Does this new paper answer, or even address, the challenges to the creative power of the selection/mutation mechanism? Does it show that the mutation/selection mechanism, or any other undirected materialistic mechanism, could generate the new genetic (and epigenetic) information necessary to produce the innovations in form and structure that occurred in the Cambrian period? It does not. Instead, at most it measures the “rate of change” that occurred within one phylum during (and after) its origin in the Cambrian. The words “rate of change” are key here. Even ignoring the paper’s other problems (which my colleague Casey Luskin will discuss in a subsequent post), Lee and his colleagues only succeed in measuring a rate at which molecular and morphological change occurred (“evolution” in the first sense) during and after the Cambrian period. The study never established that the change it measured was caused by natural selection and random mutations, or any other purely materialistic evolutionary mechanism. Thus, it does not provide a causal explanation for the origin of the animal forms that arise in Cambrian period — the absence of which constitutes the central mystery addressed in Darwin’s Doubt. What the Study DidThe study begins by using molecular and morphological data to construct phylogenetic trees of arthropods. On the assumption that degree of biological similarity reflects the degree of relatedness, these trees were constructed by comparing the morphological traits and molecular sequences of various living arthropod species, and then grouping these species according to their number of shared similarities. As is common in such studies, the length of a branch on a phylogenetic tree corresponds to the amount of change that presumably took place along that branch. In a tree derived from the comparative analysis of similar DNA sequences in different organisms, branch length corresponds to the number of nucleotide differences in the two respective molecules, and, thus, presumably, to the number of bases that have changed since the two organisms possessing these molecules diverged from a common ancestor. On a morphology-based tree, branch length would correspond to how many morphological characters have changed since the presumed divergence. The hypothetical phylogenetic tree below illustrates these conceptual relationships, with different branch lengths (representing different amounts of change) leading to three fictional living organisms A, B, and C: 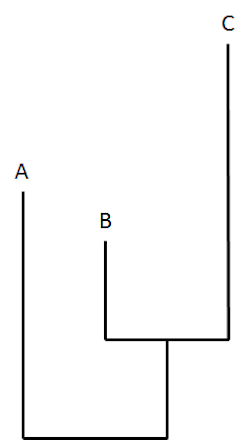
In this hypothetical tree, the length of a “branch” reflects the amount of change that has taken place during the evolution of that organism from its presumed ancestor. Here’s the same tree with fictional units of “change” added: 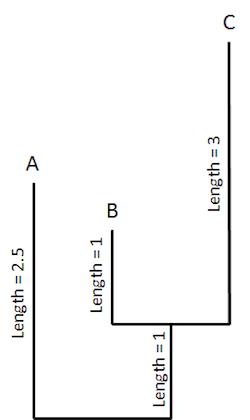
Lee and his colleagues applied this method of analysis to arthropods. Compared to many other invertebrates, arthropods have a rich fossil record. So by using fossils to date the nodes (i.e., the starting points and endpoints of branches) on their hypothetical tree, they approximated how long a given branch lasted in real time. To illustrate further, let’s assume that the first representative of a group — one that includes A, B, and C — appears in the fossil record around 400 million years ago. We’ve now dated the base of their group within the tree, labeled in blue below: 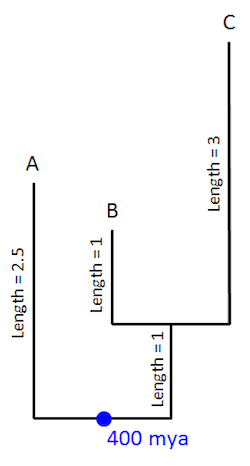
Now let’s say that the first member of the group that includes just B and C appears in the fossil record at 200 million years ago. Now we can date the split of that group as well: 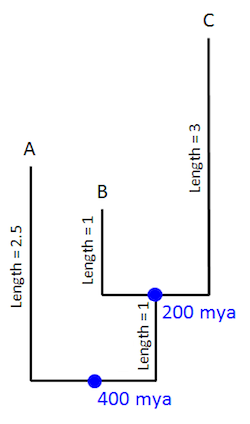
Keep in mind that the length of the branch doesn’t necessarily correspond to the amount of time elapsed. Rather, it corresponds to the amount of biological change that occurred along that branch (i.e., the number of measured molecular or morphological differences between the two groups representing the beginning and end of the branch). The length of time represented by the branch is only assigned after the fact, using fossils thought to mark the first appearance of the two groups. Nevertheless, once evolutionary biologists think they know how much change has taken place (the number of molecular or morphological differences) along a branch (between two different organisms), and how much time elapsed along the branch (i.e., between the presumed first appearance of the two organisms in question), they can then calculate a rate of evolutionary change. So now that we’ve included a couple of fossil dates in our hypothetical tree, we can start calculating rates of change along the branches that led to A, B, and C:
All this is very well. However, methods for calculating rates of change do not establish the cause of the change in question. This is axiomatic. And it is just as true of genetic or morphological change as of any other kind. For this reason, the Lee et al. paper did not establish that the emergence of animals in the Cambrian “could be explained … by way of natural selection.” Instead, it only establishes how much genetic and morphological change natural selection and random mutation (or some other cause) would need to produce in a given amount of time in order to explain the origin and evolution of arthropods. After dating the nodes of their hypothetical trees, the Lee et al. study calculated that rates of genetic and morphological change among arthropods during the Cambrian period were five times greater than they were after the Cambrian period. Fair enough. But did the authors establish that mutation and natural selection could generate the amount of change that their study measured? Did they establish that natural selection was responsible for the genetic and morphological change that had occurred within arthropods? They did not. The authors assumed that natural selection and random mutations were responsible for the change that had occurred and then simply asserted that natural selection could produce the rate of morphological change they measured. In other words, they begged the question as to the rapidity with which the mechanism of mutation and selection can produce morphological novelty. They did not demonstrate that the neo-Darwinian mechanism has the creative power to generate morphological novelty this quickly. Thus, although Lee and colleagues claim to have refuted unnamed “opponents of evolution,” they certainly did not refute the specific quantitative challenges to the creative power of the mutation selection mechanism presented in Darwin’s Doubt, which cast doubt on the ability of the neo-Darwinian mechanism to produce even modest changes or innovations in single proteins within known evolutionary deep time. In particular, Lee did not explain how random mutation and natural selection could have overcome the problem of the rarity of genes and proteins in combinatorial sequence space. Nor did his team show that the waiting times associated with the production of even a few coordinated mutations were any shorter than the prohibitively long waiting times calculated by the researchers cited in Darwin’s Doubt. For this reason, the study does not justify the claim of its punch line: “Inexplicably fast rates are not required to explain the Cambrian explosion of arthropods, even under an extreme scenario in which all divergences are compressed into the Cambrian.” The study by Lee et al. is, however, problematic for other reasons. We’ll have more to say on those later.
|
|||||
|
|

